YOOme entwickelt Ihre Medizinprodukte
Sicherheitskonzepte, Requirements Analyse, Systementwicklung, Testing, Hardware
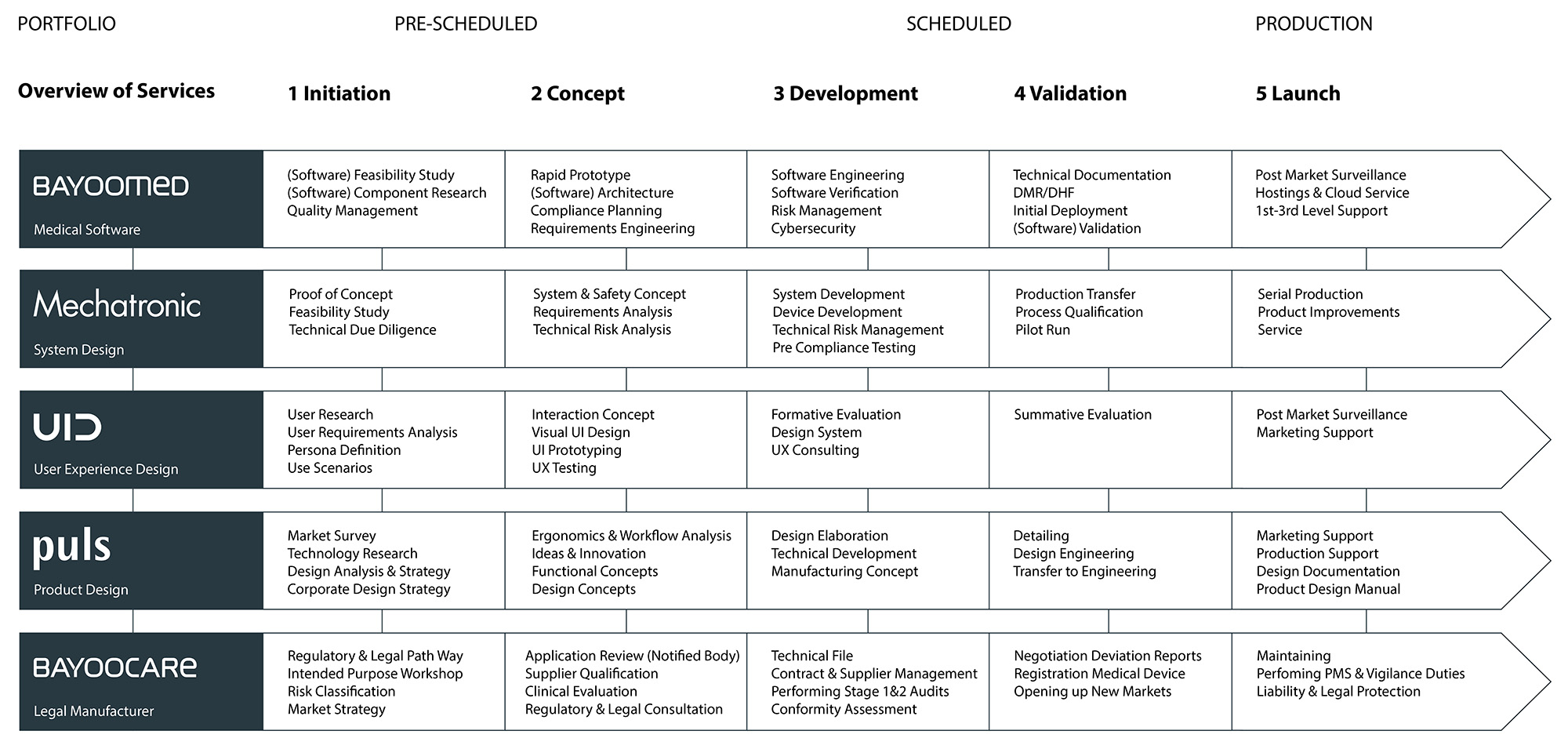
Die gesamte Medizinprodukte Journey aus einer Hand
YOOme ist der größte Unternehmensverbund für die Entwicklung Ihrer Medizinprodukte in Europa. Sie als Hersteller greifen über YOOme auf alle Leistungen der gesamten Medizinprodukte Journey zu: Ideenfindung, Konzeption und Requirements Engineering, medizinische Hard- und Softwareentwicklung, Cybersecurity, Validierung und Verifizierung, Design, Usability & UX, Inverkehrbringung und Maintenance.
Ihre YOOme Expert:innen
YOOme besteht aus den Teams der BAYOOCARE (Legal Manufacturer), BAYOOMED (Medizinische Software), Mechatronic (Entwicklung & Fertigung), Puls Produktdesign (Produktdesign & UX) und UID (User Interface Design). Alle YOOme-Partner sind Teil der 350 Mitarbeiter:innen starken BAYOONET Group und haben zahlreiche gemeinsame Projekte realisiert, Medizinprodukte entwickelt und international in Verkehr gebracht.
Der Vorteil für Sie:
Vertraute Zusammenarbeit
Durch unsere unternehmerische Verbundenheit sind wir ein eingespieltes Team und verfolgen ein gemeinsames Ziel:
Ihr Medizinprodukt ganzheitlich und sicher zu entwickeln. Bei der gesamten Medizinprodukte Journey begleitet Sie als Medizinprodukte Hersteller daher eine feste YOOme-Kontaktperson.
Die Partnerunternehmen können dabei punktuell unterstützen oder langfristiger Begleiter für Sie sein und mit der erfolgreichen Zulassung ist für YOOme noch lange nicht Schluss: YOOme ist Ihr Partner zur Inverkehrbringung in mehr als 50 Ländern, MDSAP und ISO 13485 zertifiziert und unterstützt Sie bei der Post-Market Surveillance und Vigilanz.
So unterstützen wir Sie
In einem Erstgespräch verschaffen wir uns gemeinsam einen Überblick über die Entwicklung oder Überarbeitung Ihres Medizingeräts. Wie können wir unsere Expertise am effizientesten nutzen, um Ihr Projekt zu realisieren? Sie haben die Wahl: Nutzen Sie unser komplettes Portfolio als Full Service Provider – von der Idee bis über das Ende des Medizinproduktezyklus hinaus. Oder finden Sie in uns Ihren Partner für einen Entwicklungsbereich.
Ganz egal, wo Sie stehen: Mit YOOme haben wir die richtigen Experten für Ihr Projekt.
Noch offene Fragen?